Overview of Adhesives
Share:

Image Credit: Anatolir/Shutterstock.com
At A Glance
- Introduction
- Common Types of Adhesives
- Adhesives vs. Sealants
- Basic Mechanisms Underlying Adhesives
- Characteristics of Adhesives
- Selection Considerations for Adhesives
- Applications of Adhesives
- Key Adhesive Terminology
- Summary
Introduction to Adhesives
Adhesives is an umbrella term which refers to any substances which, when applied between the surfaces of two or more materials or objects (i.e., substrates), can be used to hold, fasten, or bond them together. The durability of the attachment (i.e., the bond strength) formed between substrates by the adhesive largely depends on the particular adhesive’s properties—specifically its adhesion and cohesion. These properties are the primary mechanisms underlying adhesives; therefore, calculating the failure point of both properties for each adhesive available helps to determine the most suitable adhesive for use in an application given the technical requirements and specifications.
Adhesives are widely employed throughout industry for permanent, semi-permanent, and temporary attachment purposes in a variety of residential, commercial, and industrial applications. Some of the characteristics by which the wide range of adhesives available can be classified and categorized include load carrying capacity, chemical composition, reactivity or inertness, and form. Each of these adhesives demonstrates different properties and offers different advantages, but, as with adhesive and cohesive strengths, the suitability of a particular property or characteristic (and the respective adhesive) is dependent on the application.
This article focuses on adhesives, exploring the various classifications and categories available and explaining their respective characteristics, advantages, and disadvantages. Additionally, it outlines some of the common types employed and the selection considerations for choosing an adhesive for an application.
Common Types of Adhesives
Based on the characteristics outlined above, there are several different types of adhesives available. Some of the more common types of adhesives employed throughout industry include:
- Anaerobic adhesives: Anaerobic adhesives are acrylic-based adhesives which cure in the absence of air. The presence of metal accelerates the curing process. This type of adhesive typically has low viscosity, is available in liquid and paste solutions, and is suitable for securing, sealing, and retaining close-fitting and structural parts.
- Cyanoacrylates adhesives: Cyanoacrylate adhesives, also referred to as “instant glues”, are adhesives that cure in the presence of moisture and ultraviolet (UV) light (e.g., Krazy Glue ®, a trademark of Toagosei Co., Ltd). This type of adhesive is available in a variety of formulations ranging from low to high viscosity and is suitable for porous and non-porous substrates. It is not suitable for structural applications due to its low shear strength.
- Epoxy adhesives: Epoxies are typically available as single-part and multi-part systems. These types of adhesives demonstrate high shear and peel strength (even in extreme temperatures and environments) and are suitable for gap filling and bonding dissimilar substrates.
- Hot glue: Hot glue is a type of hot melt adhesive typically derived from thermoplastic compounds. This type of adhesive demonstrates high levels of tackiness and is suitable for use on both porous and non-porous substrates. Generally, hot glue solidifies by cooling, but some variants can be cured by the presence of moisture or UV light.
- White glue: White glue is a common polyvinyl acetate (PVA) glue traditionally used for arts and crafts purposes (e.g., Elmer’s® Glue, a trademark of Elmer's Products, Inc.). This type of adhesive requires contact and pressure during solidification and is suitable for cloth, cardboard, paper, wood, and other porous substrates.

Image Credit: Nor Gal/Shutterstock.com
Adhesives vs. Sealants
Adhesives are composed of similar materials, employ similar mechanisms, exhibit similar characteristics, and are used in some of the same applications as those of sealants. Therefore, in a discussion about adhesives, the topic of sealants invariably comes up.
As indicated previously, adhesives are substances which, by way of surface attachment, can be used to hold, fasten, or bond two or more substrates together. On the other hand, sealants are substances which, by way of surface attachment, can be used to fill the space between two or more substrates to create a seal or barrier which prevents the penetration of different elements—e.g., fluids, dust, fire, noise, etc. These definitions allude to some of the similarities between adhesives and sealants as both substances primarily make use of the property of adhesion to form surface attachments. Other overlapping characteristics include some of the composition materials, application and processing requirements, failure mechanisms, substance-to-substrate interactions, and, depending on the type of adhesive or sealant employed, capabilities for both adhering and sealing.
Despite these numerous similarities, industrial standards typically classify adhesives and sealants as two distinct and separate substances. This delineation is largely due to their differing primary functions. Whereas the primary function of adhesives is to bond two or more substrates together, sealants are primarily employed to fill a space and create a barrier between two or more objects. Although some adhesives—typically referred to as adhesive sealants—can fulfill both adhering and sealing functions, in general, their primary function is not necessarily to fill spaces or create barriers between substrates. Conversely, while some stronger sealants may qualify as adhesives, most sealants demonstrate weaker attachment and are not suitable for bonding or attachment purposes. Therefore, the following article will only focus on adhesives and will not cover sealants.
Basic Mechanisms Underlying Adhesives
As indicated previously, adhesion and cohesion are the primary mechanisms underlying adhesives. Regardless of type and design, all adhesives operate by these same fundamental properties, which describe the interactions between the adhesive, the substrate (also referred to as the adherend once the adhesive has been applied), and the substrate’s surface; the components of the adhesive; and the components of the substrate.
Adhesion
Adhesion is a measure of the attractive forces between two different substrates which bond them together. By calculating the level of adhesion demonstrated by an adhesive, one can determine the strength of the attachment formed between the adhesive and substrate and, combined with the measure of cohesive forces, the bond strength.
Several theories have been developed to define and describe this property, but no single theory has yet to accurately and comprehensively explain adhesion across the wide range of adhesives available. Some of the most common theories of adhesion include:
- Adsorption: The adsorption theory states that adhesion results from the intermolecular contact between the surfaces of two substances—i.e., between the adhesive and substrate. The attractive forces resulting from the contact—e.g., chemical bonds, van der Waals forces, etc.—hold the two substrates together.
- Mechanical interlocking: The mechanical interlocking theory states that adhesion results from the flow of the adhesive into and around the cavities and protrusions of both substrates’ surfaces. Once hardened, the adhesive mechanically holds the two substrates together.
- Interdiffusion: The interdiffusion theory states that adhesion results from the diffusion of molecules into and between the adhesive and substrate(s). In some cases, the adsorption of the adhesive’s molecules into the surface(s) of the substrate(s) can result in a chemical reaction—e.g., melting. The diffusion of molecules and subsequent hardening of the adhesive leads to the formation of a bond between the adhesive and the substrate(s) which effectively bonds together the two substrates.
- Electrostatic attraction: The electrostatic attraction theory states that adhesion results from the development of electrostatic forces at the contact point (i.e., the interface) between the adhesive and substrate given differing electronic band structures. These attractive forces resist separation—if only temporarily—which forms an attachment between the adhesive and the substrates and, consequently, between the two substrates.
Cohesion
Cohesion is a measure of the attractive forces within a substance which hold it together. This value can refer to either the strength of the bonds between the adhesive’s components or the substrate’s components. Some of the factors which may influence an adhesive’s cohesive properties are the chemical and intermolecular bonds between the atoms of the adhesive’s components and the crosslinking of molecules (from short-chain to long-chain). Within an adherend, cohesion similarly plays a role between the atoms and molecules which make up the substance.
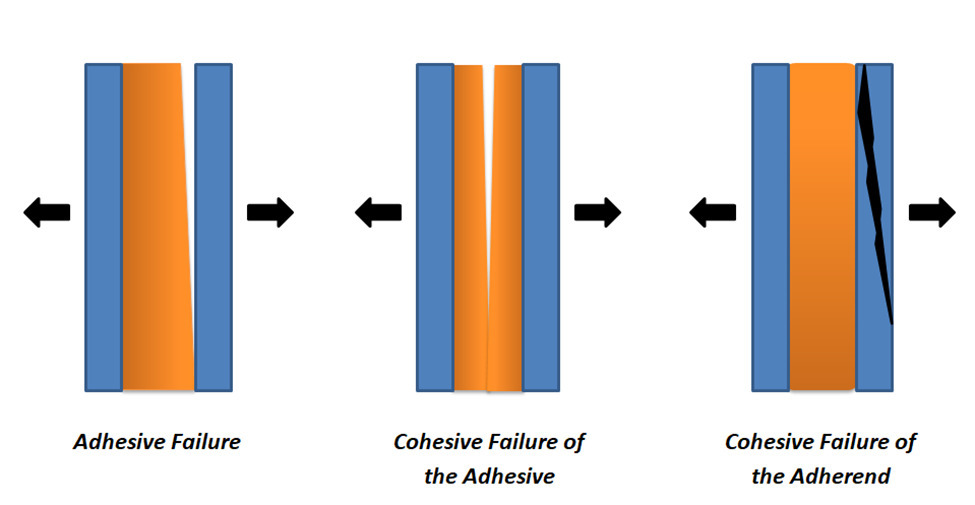
Adhesive and Cohesive Failure Modes
In most cases, the failure of an adhesive bond can be attributed to an issue with the adhesive itself, either in regard to its adhesive or cohesive properties. However, cohesive failure within the adherend can also cause an adhesive bond to fail.
- Adhesive failure occurs as a result of the inability to establish an adequate bond between the adhesive and adherend. For example, given two substrates attached by an adhesive and then pulled apart, if the adhesive remains fully attached to one substrate and not the other, this would be considered an adhesive failure.
- Within the adhesive, cohesive failure occurs when the bond of the adhesive to the adherend is stronger than the bonds between the atoms and molecules of the adhesive. For example, returning to the previous case where two adherends are separated, if some of the adhesive remains on both substrates’ surfaces this would be considered a cohesive failure of the adhesive.
- Within the adherend, cohesive failure occurs when the adhesive bond between the adhesive and the adherend and the cohesive bonds of the adhesive exceed that of the bonds between the atoms and molecules of the substrate. This condition—referred to as the cohesive failure of the adherend—typically occurs at the point of attachment created between the substrates and presents itself as the adhesive remaining intact while the substrate itself succumbs to structural fatigue—i.e., experiences some break or fracture.
The three conditions mentioned above and illustrated in Figure 1, below, represent the three main types of adhesive bond failure modes. Of these three failure modes, the cohesive failure of the adherend is the most ideal as it typically indicates the proper selection and application of the adhesive for the given substrate of given structural integrity.
Figure 1 – Adhesive Bond Failure Modes
Characteristics of Adhesives
As outlined above, all adhesives operate by the same basic properties. However, there are several different types of adhesives available, each of which can be differentiated by their particular characteristics. Some of the characteristics by which adhesives are commonly classified and categorized include:
- Load carrying capacity
- Chemical composition
- Reactivity
- Form
Adhesive Load Carrying Capacity
The load carrying capacity of an adhesive refers to the maximum load an adhesive-bonded joint can support or bear without experiencing deformation or failure of the adhesive. Depending on the load carrying capacity demonstrated by the adhesive, it can be further classified as structural, non-structural, or semi-structural.
Structural Adhesives
Structural adhesives are ones which demonstrate high bond strengths and durability and are capable of tolerating heat and solvent and supporting high loads. The shear strength of typical structural adhesives averages and exceeds 1000 psi. Due to their high load carrying capacity, these types of adhesives are used for long-term or permanent attachment purposes, such as in joining materials in construction applications.
Non-structural Adhesives
In contrast to structural adhesives, non-structural adhesives are ones which are designed for light loads and are not typically capable of withstanding long periods of environmental exposure. Due to their low load carrying capacity, these types of adhesives are typically used for short-term or temporary attachment purposes, such as holding substrates in place rather than bonding them together. However, depending on the type of non-structural adhesive employed, they also can be used in some long-term or permanent attachment applications and as secondary fasteners used alongside mechanical fasteners, such as screws or bolts.
Semi-structural Adhesives
Depending on the type employed, semi-structural adhesives are adhesives which can be suitable for structural or non-structural applications. While these types of adhesives can support higher loads than non-structural adhesives, unlike structural adhesives they are unable to support these higher loads continuously or for long periods without deformation or failure.
Some of the types of semi-structural adhesives available overlap with that of non-structural and structural adhesives and include, for example, hot melts, polyurethanes, and other polymers.
Adhesive Chemical Composition
While in the previous section, adhesives were categorized based on their load carrying capacity, this section categorizes them based on their chemical composition. The chemical characteristics by which adhesives can be classified include:
- Natural vs. synthetic
- Organic vs. inorganic
- Material
Natural vs. Synthetic Adhesives
Although historically adhesives were made solely of naturally-occurring components, today they can be composed of natural compounds or synthetic compounds.
Natural adhesives are derived from animal material, plant matter, and, in some cases, minerals.
- Animal-based adhesives are made from components such as albumin, beeswax, casein, gelatin rendered from hides, hooves, or bones, and shellac.
- Plant-based adhesives are made from components such as dextrin, natural resins (e.g., gum arabic, balsam, etc.), oils and waxes (e.g., linseed oil), soybean protein, and starch.
- Mineral-based adhesives are made from components such as amber, asphalt, paraffin, silicates, and sulfur.
Although these types of adhesives are relatively inexpensive to manufacture and continue to find application throughout industry, especially for wood, paper, film, and foil materials, the majority of the commonly employed adhesives fall in the more recently developed category of synthetic adhesives.
For the most part, synthetic adhesives are derived from human-made polymers (i.e., not naturally-occurring) including thermoplastics, thermosets, and elastomers (further information on these compounds is outlined in a later section of this article – see Adhesive Material). While more costly than natural adhesives, these types of adhesives offer greater bond strengths and durability, as well as provide more options for customization.
Organic vs. Inorganic Adhesives
Although most types of adhesives—both natural and synthetic—are made of organic compounds, organic and inorganic materials are used to manufacture and produce adhesives. In general, organic compounds are materials which contain carbon (and typically hydrogen, oxygen, or nitrogen) atoms, while inorganic compounds are materials which do not contain any carbon atoms. However, there are a few exceptions to this delineation. For example, carbon tetrachloride (CCl4)—which does not contain hydrogen, oxygen, or nitrogen—is considered an organic compound, while carbon dioxide (CO2) and carbon monoxide (CO)—which do contain carbon—are considered inorganic compounds.
Natural organic adhesives include animal-based and plant-based adhesives, while natural inorganic adhesives include mineral-based adhesives. Synthetic organic adhesives include all three of the categories mentioned previously—i.e., thermoplastics, thermoset, and elastomer adhesives—while synthetic inorganic adhesives include non-carbon-based adhesives, such as cement and mortar.

Image Credit: Bilanol/Shutterstock.com
Adhesive Material
As outlined above, there are several different types of compounds from which adhesives are derived, including acrylic, neoprene, polyurethane, silicone, and urethane. These various compounds can be categorized into larger umbrella groups, with the most commonly employed being synthetic materials, such as:
- Thermoplastic adhesives: Thermoplastic adhesives do not require a curing period. Instead, once the adhesive has been applied, it either cools (e.g., in the case of hot melt adhesives) or dries (e.g., in the case of glue) to form an adhesive bond between substrates.
As thermoplastic materials do not require a curing period, they do not undergo a physical or chemical change upon the application of heat. Therefore, they can be remelted and recycled for future attachment applications. While this ability offers some advantage in regard to long-term adhesive costs, it also limits the suitable operating temperatures and the environmental conditions (specifically chemical and solvent exposure) for thermoplastic adhesives.
Some of the compounds from which thermoplastic adhesives are made include cellulose derivatives, polyacrylates, polyethers, polysulfones, saturated polyesters, and vinyl polymers and copolymers. - Thermosetting adhesives: Unlike thermoplastic adhesives, thermosetting adhesives do require a curing period. When first applied to a substrate, thermoset adhesives have short polymer molecules. However, during the curing process, these adhesives undergo an irreversible chemical cross-linking reaction, which bonds together long chains of polymer molecules. This reaction changes the physical properties and chemistries of thermoplastic adhesives, allowing them to harden and the adhesive bond to set. Depending on the type of thermosetting adhesive employed, there are several methods of curing it, including the application of heat and pressure, or exposure to moisture, radiation, or a catalyst.
As thermosetting adhesives require a curing period for the adhesive bond to set, these types of adhesives cannot be sufficiently softened for re-positioning or adjustment nor reused for future attachment applications. Additionally, while they typically express greater resistance to heat and solvents and provide higher bond strengths than thermoplastic adhesives (making them suitable for structural applications), extreme temperatures may still result in the degradation or weakening of the adhesive bond.
Some of the compounds from which thermosetting adhesives are made include amino plastics, epoxies, furanes, phenolic resins, polyaromatics, and unsaturated polyesters. These adhesives are available in several forms, including liquids, pastes, and solids, and single- or multi-part solutions. - Elastomeric adhesives: Elastomeric adhesives, such as rubber adhesives, can be made from natural or synthetic elastomers. Due to these compounds’ natural elasticity, durability, and capability to withstand extension and compression stresses, these types of adhesives are suitable for attachment applications requiring high bond strength, especially for uneven loads.
Elastomeric adhesives are available in several forms, including liquids, pastes, tapes, and single- or multi-part solutions. Depending on the composition of the type employed, a curing period may be required to set the adhesive bond. - Hybrid adhesives: Hybrid adhesives are derived from an amalgamation of thermoplastic, thermosetting, and elastomeric compounds which exhibit some combination of the chosen materials’ advantageous characteristics. For example, a hybrid adhesive can express the resistance to peel and impact demonstrated by elastomeric adhesives, as well as the high resistance to heat and solvents demonstrated by thermosetting adhesives. These adhesives are available as liquids, films, and single- or multi-part solutions.
- Additives: In addition to the standard compounds found within a specific type of adhesive (as outlined above), certain attachment applications may also require additives to enhance the adhesive’s qualities. For example, colorants (such as dye or pigments) can be added for aesthetic purposes, plasticizers can be added to increase flexibility, and fillers (such as mica, alumina, silica, etc.) can be added to extend the formula and improve specific performance characteristics.

Image Credit: noprati somchit/Shutterstock.com
Adhesive Reactivity
The reactivity of an adhesive refers to the method by which the adhesive solidifies and the adhesive bond sets. Depending on the adhesive employed, there are several different solidification methods, including:
- Chemical reaction
- Loss of solvent
- Cooling
Solidification by Chemical Reaction
With regards to adhesives, chemical reaction, depending on the adhesive employed, refers to the adhesive’s reaction to a curing agent or another catalyst, such as heat, moisture, ultraviolet (UV) radiation, and lack of oxygen.
Adhesives which solidify by chemical reaction are, by nature, thermosetting. Therefore, they generally express high bond strengths, offer significant temperature and solvent resistances, and are suitable for structural and non-structural applications. Additionally, they can be used for bonding substrates with larger surface areas.
Chemically-reactive adhesives are available in several forms, including single-part and multi-part solutions, as well as liquids, pastes, tapes, films, and powders. Some examples of this type of adhesive include acrylic, anaerobic, cyanoacrylates, and epoxy adhesives.

Image Credit: Toagosei America, Inc.
Solidification by Solvent Loss
Solvent-based adhesives, such as water-based latexes or other water-based dispersions, solidify due to the evaporation or diffusion of the solvent. Solvents serve as carrier materials within these types of adhesives, lowering their viscosities and allowing for their application onto the substrate. Once a solvent-based adhesive is applied onto a substrate, the solvent evaporates from the adhesive into the atmosphere or diffuses into the substrate, allowing viscosity to increase, the adhesive to dry, and eventually, the adhesive bond to set.
These types of adhesives are available in several forms, including
- Contact adhesives: Typically applied through spray or roll coating, contact adhesives are adhesives of high cohesive strength which must be applied to both substrates to form an adhesive bond. Once applied to the substrates, the adhesive is subjected to a drying period in which a portion of its solvent is evaporated (in some cases, with the addition of heat) to develop the adhesive’s tackiness. Once the optimal level of tackiness is attained, there is a short window of time in which the two adherends must be brought into contact. Within this window, the properties of the adhesive and the application of pressure allow the adhesive bond to form.
- Pressure-sensitive adhesives (PSAs): While pressure-sensitive adhesives are applied similarly to contact adhesives (i.e., applied to a substrate and allowed to dry), unlike contact adhesives they exhibit permanent tackiness, even after complete solidification. Because of this quality, these types of adhesives can be pre-applied to a substrate (e.g., film, form, or other backing material) and dispensed as needed. One common example of pressure-sensitive adhesives is adhesive tapes.
- Resinous solvent adhesives: Resinous solvent adhesives, also known as resin adhesives, are adhesives which are typically applied to porous substrates. The porosity allows these adhesives to flow into and around the cavities and protrusions of the substrates. As the solvent evaporates or diffuses into the substrate, the adhesive hardens and mechanically interlocks the two substrates together.
- Reactivatable adhesives: Reactivatable adhesives, also known as solvent-activated adhesives, are adhesives which are pre-applied to a substrate and allowed to dry to a non-tacky state for storage and shipment purposes. As needed, these adhesives can be re-activated (i.e., made to be tacky again) by the application of a solvent, which allows substrates to be bonded together given contact and pressure. One common example of this type of adhesive is moisture-activated stamps.

Image Credit: Mehmet Cetin/Shutterstock.com
Solidification by Cooling
Solidification by cooling occurs in solid adhesives (typically thermoplastic-based adhesives) which are subjected to melt conditions. Melting the adhesives allows them to soften, flow out across, and be applied to the substrate(s). After application, the melted adhesive cools and hardens, forming a bond between substrates. One example of adhesives which employ this heat-and-melt application method is hot glue.

Image Credit: nanantachoke/Shutterstock.com
Adhesive Form
As outlined above, there are several different classifications and categories of adhesives, each of which exhibits different properties and characteristics. Depending on the adhesive employed, they are also available in various physical forms, including:
- Liquid (solventless)
- Paste (solventless)
- Solvent-based
- Solid
Both liquid and paste adhesives are available in single-part or multi-part solventless solutions. Solvent-based adhesives generally come in liquid form but are classified separately from the general liquid adhesives category as they require additional conditions for use—i.e., materials and an environment which are conducive to solidification by evaporation or diffusion (solvent loss). Solid adhesives are available in a variety of different forms, including sheets, powders, and various shapes and preforms.
Table 1, below, outlines some of the characteristics of adhesives by form, as well as provides some examples.
Table 1 – Characteristics and Examples of Adhesives by Form
|
Form |
Characteristics |
Example(s) |
|
Liquid |
|
|
|
Paste |
|
|
|
Solvent-based |
|
|
|
Solid |
|
|
|
Sheet |
|
|
|
|
|
|
Shape/Preformed |
|
Selection Considerations for Adhesives
While there are several types of adhesives available, the suitability of each type for attaching or bonding substrates is dependent on the specifications and requirements of the application. Some of the factors that industry professionals should keep in mind when choosing an adhesive for their application include:
- Application: The specifications and requirements of the attachment application largely determine the classifications of adhesives most suitable for use. For example, if the application requires support for heavy loads, a structural adhesive may be required; while if the application is mainly aesthetic or involves lighter loads, a non-structural or semi-structural adhesive may suffice. Some of the other application requirements to keep in mind are the types of stress the adhesive needs to withstand (e.g., shear, tensile, compressive, peel, cleavage, etc.) as these can influence if and when an adhesive bond fails.
- Environment: The environmental conditions experienced by an adhesive can affect weaken the strength of an adhesive bond. Some of the environmental factors to consider which can influence the characteristics and performance of an adhesive include temperature, moisture, weather, radiation, acidity and alkalinity, and bio-agents. Depending on the environmental conditions, adhesives capable of withstanding them are available, such as fire-resistant glue and high-temperature adhesives.
- Production: The stages of production involving adhesives include not only the application and curing stages, but the storage, handling, and disposal stages as well. Some of the production factors to keep mind include the adhesive’s shelf life and working life, the conditions and methods necessary for application and—if applicable—curing, and the standards and protocols required for safe use and disposal.
- Substrate material: In order to properly establish an adhesive bond, the adhesive must be compatible with the substrate(s)—i.e., the adhesive is attracted and can adhere to the substrate(s) via one or more of the methods of adhesion. Some adhesives are even classified by the type of substrates for which they are compatible. For example, foam bonding adhesives are used for bonding foam substrates together, metal bonding adhesives for bonding metal substrates together, and rubber to metal bonding adhesives for bonding rubber to metal substrates.
- Cost: The cost of an adhesive includes not only the initial price of the substance, but the cost of labor, the dispensing, application, and (if applicable) curing equipment, and the curing and processing time (downtime) as well. While it is necessary to choose an adhesive which effectively fulfills the requirements of the application, it is also important to consider the overall costs of the chosen adhesive to better determine whether it is worth the investment.
- Other: Beyond those mentioned above, other factors to consider include the dimensions of the bonding area and standard or quality requirements.
Applications of Adhesives

Image Credit: Glue Dots International
Adhesives are used throughout industry for bonding and attaching a variety of substrates. Several types of adhesives are available and are employed across (and categorized by) a wide range of industries and applications, including:
- Aerospace adhesives
- Apparel, clothing, and garment adhesives
- Appliance adhesives
- Automotive adhesives
- Cloth, fabric, and textile adhesives
- HVAC adhesives
- Medical device adhesives
- Optical adhesives
- Packaging adhesives
Key Adhesive Terminology
Adherend: The term used to refer to the substrate once the adhesive has been applied
Adhesion: The measure of the strength of the attachment between the adhesive and substrate
Adhesive Failure: The term used to describe the failure of an adhesive bond as a result of the inability to establish an adequate bond between the adhesive and adherend
Bond Strength: The measure of the durability of the attachment based on the cumulative forces which resist the separation of two substrates bonded by an adhesive—including adhesion and cohesion—and the total amount of stress—including tension, compression, peel, shear, etc.—required to overcome those forces
Cohesion: The measure of the strength of the attachment within the adhesive (i.e., the chemical and intermolecular bonds between the adhesive’s components)
Cohesive Failure: The term used to describe the failure of an adhesive bond as a result of the strength of the bond between the adhesive to the adherend exceeding the strength of the bonds between the atoms and molecules of the adhesive (cohesive failure of the adhesive) or the adherend (cohesive failure of the adherend).
Interface: Within an adhesive joint, the area of contact between the adhesive and the adherend
Joint: The location at which two substrates are bonded together by an adhesive
Shelf Life: The amount of time an adhesive can be stored after manufacturing and remain viable for attachment applications
Substrate: The material or substance to which the adhesive is applied
Working Life: The amount of time between the mixing and metering of an adhesive and the point at which it is no longer viable for attachment applications
Summary
This guide provides a basic understanding of adhesives, the types available, and considerations for use.
For more information on related products, consult Thomas guides and white papers or visit the Thomas Supplier Discovery Platform, where you will find information on over 500,000 commercial and industrial suppliers.
Sources
- https://www.britannica.com/technology/adhesive
- http://www.newworldencyclopedia.org/entry/Adhesive
- https://science.jrank.org/pages/91/Adhesive.html
- https://www.adhesives.org/adhesives-sealants/science-of-adhesion
- https://www.adhesives.org/adhesives-sealants/adhesive-selection/types-of-glue-glue-tips
- https://www.gvsu.edu/arttech/glue-and-adhesives-12.htm
- https://d-lab.mit.edu
- https://polymerinnovationblog.com/wp-content/uploads
- http://users.fs.cvut.cz/libor.benes/vyuka/lepeni/Handbook
- https://www.adhesives.org/adhesives-sealants/science-of-adhesion/adhesion-cohesion
- http://theadhesivesexpert.com/adhesives-failure-epoxy-failure/
- http://www.uobabylon.edu.iq
- http://www.adhesionassociates.com/papers
- https://www.pharosproject.net/uploads/files/sources/1828/ASCouncil-AdhesiveGlossary.pdf
- https://books.google.com/books
- https://info.aronalpha.net/blog/a-starters-guide-to-automobile-super-glue
- https://soichem.com/how-is-non-toxic-glue-beneficial/


